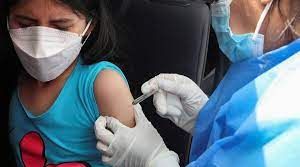
Pfizer Inc and Germany's BioNTech SE are looking forwards to submitting an emergency use authorization request on Tuesday to the US Food and Drug Administration (FDA) for their COVID-19 vaccine for children aged six months to 5 years.
In line with the pharma companies' plan, COVID-19 vaccines for children younger than 5 years could be made available by end-February. FDA can potentially give authorisation to the pharma companies' two-shot vaccination regimen in the coming weeks, the Washington Post said in its report on Monday citing people briefed on the situation.
The report added that the FDA has called on the companies to submit the application in order to allow the regulators to review the data on the two-shot vaccine.
"The idea is, let's go ahead and start the review of two doses. If the data hold up in the submission, you could start kids on their primary baseline months earlier than if you don't do anything until the third-dose data comes in," the report said, quoting people familiar with the situation.
Earlier in January, Pfizer had said latest results of the clinical trial for the vaccine for kids under the age of 5 were expected by April. The pharma company had amended its study to give a third dose to those under five after at least eight weeks since their last vaccination. Reportedly, the company amended its study as children between the ages of 2 and 4 who were given two 3-microgram doses of the vaccine did not develop the same immune response as those with a larger dose of the vaccine among older children.
 AR
AR UR
UR