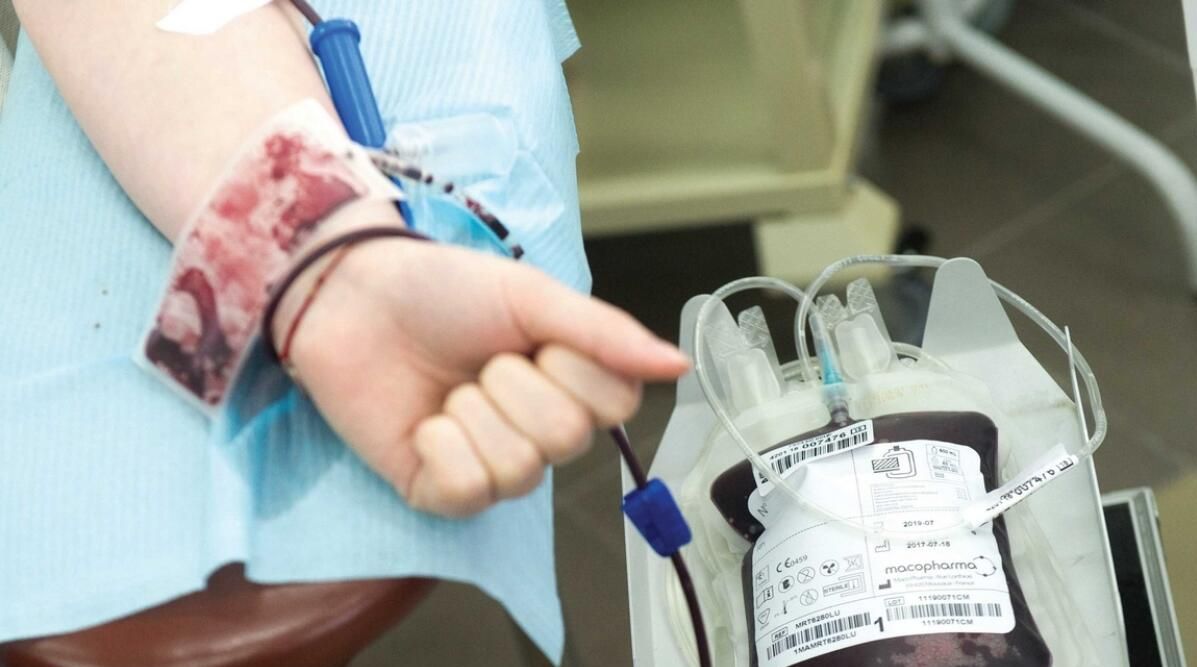
A private hospital in Abu Dhabi has agreed to participate in Phase 3 worldwide clinical trials for a potential first-in-class thalassemia treatment.
Thalassemia is an inherited blood condition that impairs the production of haemoglobin and red blood cells in the body. It is one of the most common genetic disorders in the world, and it is extremely frequent in the region.
Burjeel Medical City in Abu Dhabi is set to start two Phase 3 clinical studies of Mitapivat, a novel enzyme-activating medication (AG-348). The novel medicine directly influences red blood cell survival and has previously shown promise in prior trials for altering thalassemia treatment by addressing the disease's hallmark - chronic haemolytic anaemia.
Dr. Khaled Musallam, group chief research officer at VPS Healthcare and primary investigator on the trials, told Khaleej Times, "The Phase 3 programme will be done in conjunction with various health centres in the US and Europe."
He explained that Mitapivat would be tested in two kinds of thalassemia with very different clinical demands in the Phase 3 trial.
"One trial will be conducted in transfusion-independent patients with the goal of increasing haemoglobin levels and improving quality of life, while the other will be conducted in transfusion-dependent patients with the goal of lowering transfusion requirements and thus lowering the disease's burden on the patient and healthcare system," he added.
Advances in thalassemia management will be beneficial to the public health sector as a whole, as well as patients and their families.
More than 400 volunteers will participate in the global studies, including 240 transfusion-dependent and 171 non-transfusion-dependent thalassemia adult patients.
"We will rely on regular recruitment channels in the UAE, such as our broad healthcare network and referrals from colleagues who care for thalassemia patients," says the company.
The two trials, according to Dr Musallam, will have core treatment periods of 24 and 48 months, respectively, and will last up to five years.
"We are always ready to bring on cutting-edge technologies and recruit brilliant physicians and scientists to our medical facilities to give state-of-the-art treatment alternatives for patients," said John Sunil, CEO of Burjeel Hospitals in Abu Dhabi. This is especially important for rare diseases, which frequently have limited access to optimum management and long-term holistic care."
Meanwhile, the hospital and the HH Sheikh Sultan Bin Khalifa Al Nahyan Humanitarian and Scientific Foundation announced a partnership last month to improve thalassemia patient treatment.
The Ambassador of Cyprus to the UAE, Yannis Michaelides, said that the two countries have a strong bilateral relationship on thalassemia therapy.
"The Republic of Cyprus and the UAE's beneficial collaboration in combating thalassemia syndromes dates back to the foundation of the Emirates Thalassemia Society and continues to this day on a high note with the active backing of our two nations' leadership," said the ambassador to Khaleej Times.
Both the UAE and Cyprus, according to Michaelides, have gone above and above to assist other impacted countries.
"The Thalassemia International Federation — a global umbrella organisation of 120 thalassemia patient organisations from 57 countries throughout the world – is headquartered in Cyprus," he added.
“We work together to raise awareness and provide support to all afflicted countries throughout the world," Michaelides stated, adding that Cyprus and the UAE have a shared desire to help persons with thalassemia that reaches beyond our borders.
 AR
AR UR
UR