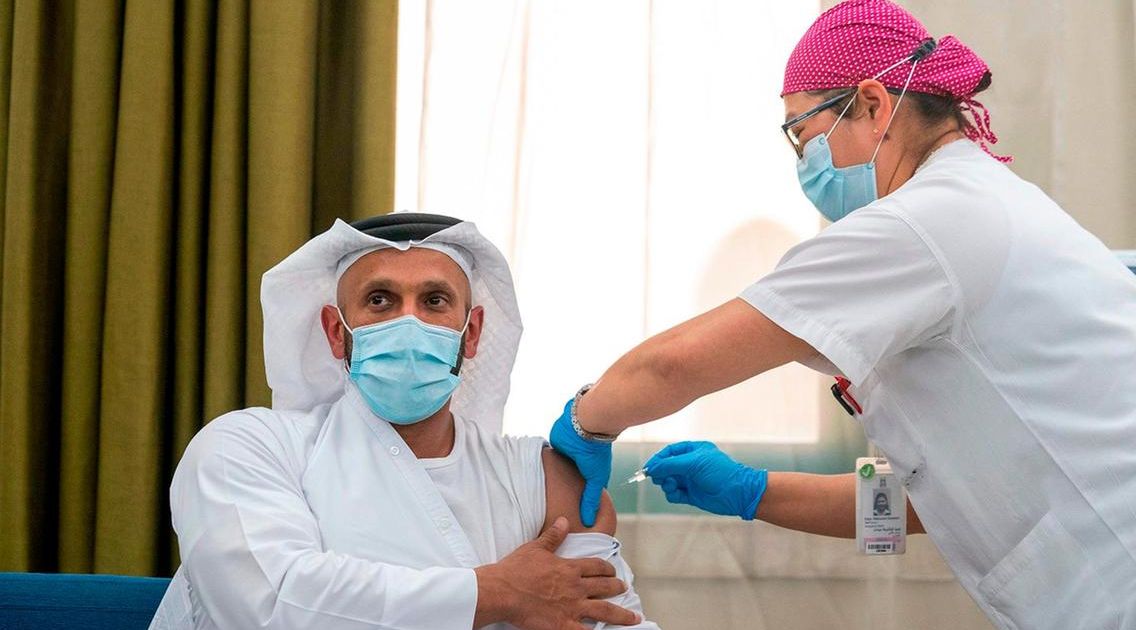
The UAE health authorities have successfully initiated the Phase III of the landmark clinical trials of an inactivated vaccine for COVID-19, with the Abu Dhabi health chief receiving his second shot of the vaccine.
Last month, Sheikh Abdullah bin Mohammed Al Hamed, the chairman of the Department of Health Abu Dhabi, volunteered to be the first patient to test the inactive COVID-19 vaccine.
Overseeing the Phase III trials, acting undersecretary department Dr Jamal Al Kaabi was the second person to volunteer for the vaccine programme. Recently, the two men received their second shots as part of the clinical trials.
According to the rules of the programme, the volunteers are required to visit the testing centres at least 17 times in over roughly 42 days. Furthermore, they are restricted to travelling abroad during the span of the programme. Moreover, after completion of the trial, volunteers have to be available for regular telephonic consultations for up to six months.
Sharing the experience after the first vaccine shot, Sheikh Abdullah affirmed that the volunteers did not feel any side effects. He added that the authorities require around 15,000 volunteers to conclude the scientific research phase and to progress towards launching the vaccine to the market. At the same time, volunteers must be aged between 18 to 60 and in good health.
"After having the first dose of the vaccine, we felt no side effects at all," said Sheikh Abdullah.
volunteered as the first patient to test the inactive vaccine, which contains a killed version of the germ that causes Covid-19, last month.
Significantly, the UAE launched the world's first Phase III clinical trial for a COVID-19 vaccine in Abu Dhabi in July. As per reports, the vaccine contains a killed version of the germ that causes COVID-19. Led by Abu Dhabi's Health Department, the Phase III clinical trials are being conducted by Abu Dhabi-based Group 42 in partnership with Chinese drug maker Sinopharm.
Earlier this week, Abu Dhabi authorities also set up a new walk-in facility at the Abu Dhabi National Exhibition Centre (ADNEC) that provides free screening and testing services to up to 1,000 potential volunteers for the programme in a day. All those willing to volunteers for the clinical trials in Abu Dhabi can get themselves registered at 4humanity.ae or can call at 02 819 1111.
 AR
AR UR
UR